
The initial step of the solar-energy conversion to chemical energy is performed in the photosynthetic reaction center (PSRC) of green plants and some bacteria. The functions of the PSRC are of global significance and offer quite challenging subjects to be clarified by means of science and technology.
There are several questions to be clarified by quantum chemists:

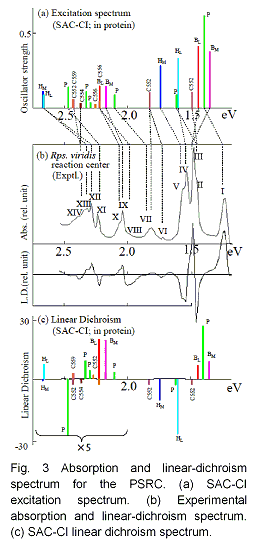
The excitation spectrum of the photosynthetic reaction center (PSRC) of Rps. Viridis is assigned by using the SAC (symmetry adapted cluster) - CI (configuration interaction) method. All the chromophores included in the PSRC,
were calculated within the environment of proteins, waters, and the other chromophores which were dealt with by the point-charge electrostatic model (Fig. 3). We have assigned successfully all the peaks in the experimental spectrum in the energy range from 1.2 to 2.5 eV. The assignment was done by comparing the SAC-CI theoretical spectrum with the experimental one in excitation energy, oscillator strength, linear dichroism data (angle of transition moment), and other experimental information available. Almost all the peaks were red shifted due to the effect of proteins. The present assignment of the spectrum would give a basis for future photoexperimental studies of the PSRC.
The electronic mechanism and the origin of the unidirectionality of the electron transfer from photoexcited special pair to bacteriopheophytin in the photosynthetic reaction center (PSRC) of Rps Viridis are studied theoretically by using the SAC-CI method. The effects of the surrounding proteins are considered by using the point charge model. The L-branch selectivity of the electron transfer is explained by the asymmetry of the transfer integral, an electronic factor, which originates from a small structural asymmetry of the PSRC: the L-side chromophores are locally closer than the M-side ones, though the average separations are almost the same.The smallness of the charge recombination rate is attributed to the difference in the electron localization between the LUMO and HOMO of special pair. Protein effects on the unidirectionality are quite small as far as the electrostatic model is valid, though the proteins keep the three-dimensional arrangement of the chromophores in the PSRC. A mutation experiment for realizing M-side selectivity is suggested.

The electronic factor in the ET rate constant was also studied for the PSRC of Rhodobactor (Rb.) sphaeroides. It is a similar photosynthetic bacterium which also shows the unidirectional ET in the PSRC. The calculated electronic factor using the SAC-CI wave functions are shown in the figure below. The origin of the unidirectionality would be in the ET from bacteriochlorophyll(B) to bacteriopheophytin (H), not from the special pair (P) to B as in the Rps. viridis: The electronic factor of the A-branch ET is 20 times larger than that of the B-branch. An analysis clarified that the unidirectionality originates from the inter-chromophore distances. This analysis also indicates that the hyperconjugations of the methyl groups with the p-electrons of the chromophores have primary contributions to the electronic factor.

The energetics of electron transfer from HL to UQ was studied using the density functional theory (DFT). In gas-phase calculations, HL had the greatest electron affinity among the three chromophores: H, menaquinone (MQ), and ubiquinone (UQ). However, the order of the electron affinity was reversed to be UQ > MQ > H by including residues that directly interact with the chromophores through hydrogen bonding. Based on the QM/MM optimized geometries, cluster models for the binding sites were constructed. The computed reaction energy was comparable to values obtained experimentally. The reaction energy can be decomposed into a vertical electron affinity term and a relaxation energy term using a driving force analysis. The most important term was the vertical electron affinity of the chromophores. Based on optimization, there was little structural reorganization. The present results indicate that, with regard to the energetics of electron transfer, local interactions between the chromophores and proteins play a decisive role by tuning the electron affinity of the chromophores, whereas the effects of distant residues are of secondary importance.

Through out these studies, we determined the electron pathway within the PSRC of Rps. viridis as indicated in the figure.

[1] Excited States and Electron Transfer Mechanism in the Photosynthetic Reaction Center of Rhodopseudomonas Viridis: SAC-CI Study, H. Nakatsuji, J. Hasegawa, and K. Ohkawa, Chem. Phys. Lett., 296 (5,6), 499-504 (1998).
[2] Excited States of the Photosynthetic Reaction Center of Rhodopseudomonas Viridis: SAC-CI Study, J. Hasegawa, K. Ohkawa, H. Nakatsuji, J. Phys. Chem. B, 102 (50), 10410 ? 10419 (1998).
[3] Mechanism and Unidirectionality of the Electron Transfer in the Photosynthetic Reaction Center of Rhodopseudomonas Viridis: SAC-CI Theoretical Study, J. Hasegawa and H. Nakatsuji, J. Phys. Chem. B, 102 (50), 10420-10430 (1998).
[4] Mechanism and Excited States and Electron Transfer Mechanism in the Photosynthetic Reaction Center of Rhodobactor Sphaeroides: SAC-CI Theoretical Study, J. Hasegawa and H. Nakatsuji, Chemistry Letters. 34, 1242-1243 (2005).
[5] Energetics of the Electron Transfer from Bacteriophephytin to Ubiquinone in the Photosynthetic Reaction Center of Rhosopseudomonas Viridis: Theoretical Study, J. Hasegawa, M. Ishida, and H. Nakatsuji, J. Phys. Chem. B, 107, 838-847 (2003).