
Since the discovery of green fluorescent protein (GFP), many studies have examined its photo-physical and -chemical properties, not only to better understand the unusual optical spectroscopic characteristics of the GFP chromophore (GFPC), but also to find a wide range of applications in molecular genetics, biochemistry, and cell biology. The GFP of the jellyfish Aequorea victoria is made up of 238 amino-acid residues and produces a greenish fluorescence (lmax 508 nm). The absorption spectrum of wildtype GFP consists of two broad peaks at 478 (2.60 eV) and 398 nm (3.13 eV). Because the ratio of the two peaks depends on the pH, temperature, and ionic strength, the two peaks at 398 and 478 nm can be ascribed to two different ground states, A- and B-forms, respectively, which differ with regard to their protonation state. Emission from the A*- and B*-forms is observed, respectively, at 420-470 nm (2.64 -2.95 eV) and 482 nm (2.57 eV).

Two ground-state protonation forms causing different absorption peaks of the green fluorescent protein chromophore were investigated by the quantum mechanical SAC/SAC-CI method with regard to the excitation energy, fluorescence energy, and ground-state stability. The environmental effect was taken into account by a continuum spherical cavity model. The first excited state, HOMO-LUMO excitation, has the largest transition moment and thus is thought to be the source of the absorption. The neutral and anionic forms were assigned to the protonation states for the experimental A- and B-forms, respectively. The present results support the previous experimental observations.
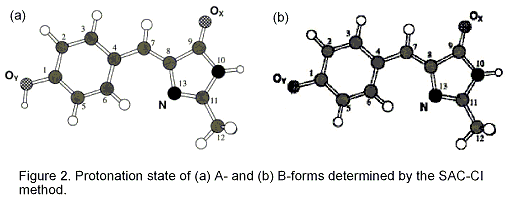
Excited states of fluorescent proteins were studied using Symmetry-Adapted Cluster-Configuration Interaction (SAC-CI) method. Protein-environmental effect on the excitation and fluorescence energies was investigated. In Green Fluorescent Protein (GFP), the overall protein-environmental effect on the first excitation energy is not significant. However, glutamine (Glu) 94 and arginine (Arg96) have the red-shift contribution. The excited states of GFP active site (GFP-W22-Ser205-Glu222-Ser65) were also calculated. Such large-scale SAC-CI calculations were performed with an improved code containing a new algorithm for the perturbation selection.Å@The SAC-CI results indicate that a charge-transfer (CT) state locates at 4.19 eV, which could be related to the channel of the photochemistry as indicated in a previous experimental study. We also studied the excitation and fluorescence energies of Blue Fluorescent Protein (BFP), Cyan Fluorescent Protein (CFP), and Y66F. The SAC-CI results are very close to the experimental ones. The protonation state of BFP was determined. Conformation of CFP indicated by the present calculation agrees to the experimentally observed structure. The result suggests that the fluorescence energy of the fluorescent proteins under the study are controlled by the electronic structures of the chromophores theirselves.

[1] Electronic Excitations of the Green Fluorescent Protein Chromophone in its Protoonation States: SAC/SAC-CI Study, A.K. Das, J. Hasegawa, T. Miyahara, M. Ehara and H. Nakatsuji, J. Comp. Chem., 24, 1421-1431 (2003).
[2] Excited States of GFP Chromophore and Active Site Studied by the SAC-CI method: Effect of Protein-environment and Mutations, J. Hasegawa, K. Fujimoto, B. Swerts, T. Miyahara, and H. Nakatsuji, J. Comp. Chem. 28, 2443-2452 (2007).