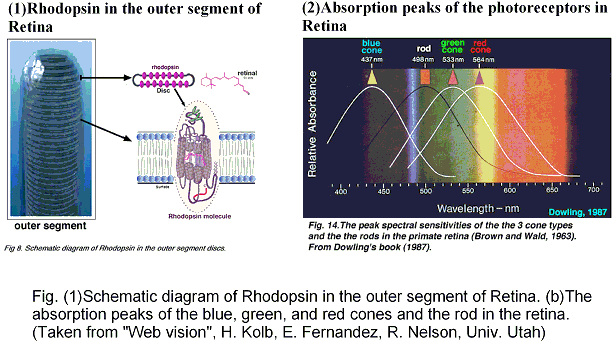
Photo-absorption is the initial event of vision, photosensing, and ion-pumps in retinal proteins. The absorption maxima of the retinal proteins are well regulated in wide spectral range to furnish the photo-receptors with color sensitivity, whereas the proteins include a common identical chromophore, retinal. The electronic excitation energy of retinal is, therefore, controlled by interaction with the apoprotein, opsin.

The SAC-CI and QM/MM methods were applied to calculations of the absorption peaks of the retinal proteins, bovine rhodopsin (Rh), bacteriorhodopsin (bR) in BR, K, and KL states, and sensory rhodopsin II (sRII). The results nicely agree with the experimental excitation energies and provided an insight into the mechanism of the large blue shifts in Rh and sRII from BR: geometric distortion in Rh and protein electrostatic effect in sRII. These results indicate that the present approach is useful for studying the excitation spectra and the mechanism of the color tuning in the retinal proteins.
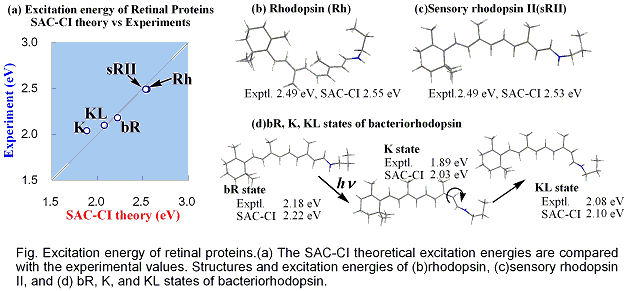
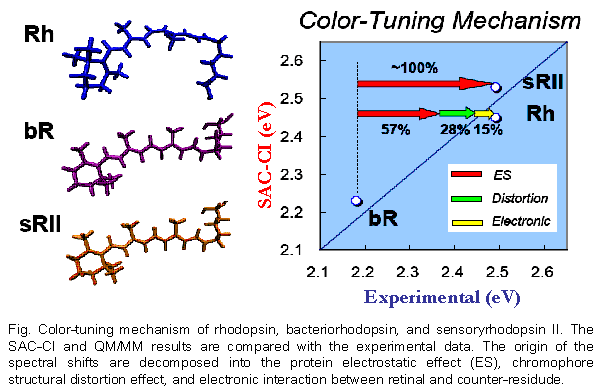
The excited states of the three retinal proteins, Bovine rhodopsin (Rh), bacteriorhodopsin (bR), and sensory rhodopsin II (sRII) were studied using the Symmetry-Adapted Cluster-Configuration Interaction (SAC-CI) and combined Quantum Mechanical and Molecular Mechanical (QM/MM) methods. The computed absorption energies are in good agreement to the experimental ones for all three proteins. The spectral tuning mechanism was analyzed in terms of three contributions: molecular structures of the chromophore in the binding pockets, electrostatic (ES) interaction of the chromophore with the surrounding protein environment, and quantum-mechanical effect between the chromophore and the counter-ion group. This analysis provided an insight into the mechanism of the large blue shifts in the absorption peak position of Rh and sRII from that of bR. Protein ES effect is primarily important both in Rh and in sRII, and the structure effect is secondary important in Rh. The quantum-mechanical interaction between the chromophore and the counter-ion is very important for quantitative reproduction of the excitation energy. These results indicate that the present approach is useful for studying the absorption spectra and the mechanism of the color tuning in the retinal proteins.
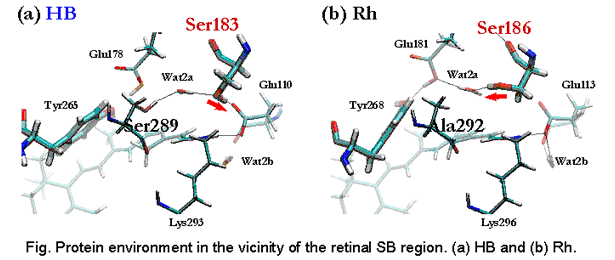
Color-tuning mechanism of Human-Blue pigment, visual color-receptor in the cone cells of the eye, has been investigated. Based on a previous Homology-Modeling structure and experimental evidences, a working model was constructed, and the structure has been optimized by QM(B3LYP)/MM(AMBER) method. SAC-CI calculation was performed to obtain photo-absorption energy. The calculated absorption energy reasonably agrees with the experiment. A decomposition analysis was performed and compared with the case of Bovine rhodopsin. The electrostatic effect from the opsin is primarily important for the color-tuning. Actually, the counter-residudes give minor contributions to the color-tuning. The blue-shift originates from other several small contriburions. The OH group in Ser183 in HB creates negative electrostatic potential around the Schiff-base region of the retinal. The direction of the OH group is controlled by a hydrogen-bonding network.
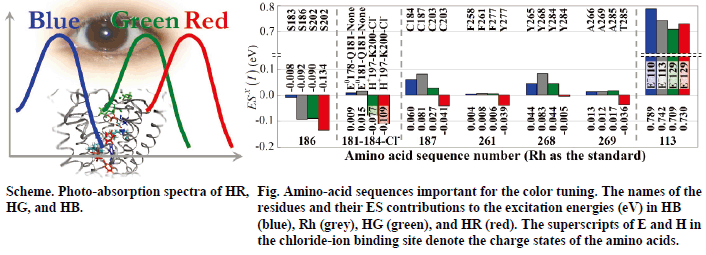
Human color vision is controlled by the red, green, and blue cone pigments. Their photoabsorption wavelengths spread uniquely over the three primary colors, although these pigments include common chromophore, retinal. In this study, molecular mechanism of color tuning in the cone pigments was clarified. The protein effect represented by the electrostatic potential is primarily important for the spectral tuning among the pigments. The structural distortion effect of the retinal chromophore is important in the human blue pigment.
The result of the analysis indicates that amino acids at specific positions in the opsins regulate the color tuning. For example, position 186 works for discriminating the HB from the others. Although all the visual pigments have serine in this position, those in Rh, HG, and HR cause electrostatic red-shift contribution to the excitation energy. The chloride-ion binding site provides red-shift contribution in HG and HR, but not in HB and Rh.
[1] Mechanism of Color-Tuning in Retinal Proteins: SAC-CI and QM/MM Study, K. Fujimoto, J. Hasegawa, S. Hayashi, S. Kato, H. Nakatsuji, Chem. Phys. Lett., 414, 239-242 (2005).
[2]Theoretical Studies on the Color-Tuning Mechanism in Retinal Proteins, K. Fujimoto, S. Hayashi, J. Hasegawa, and H. Nakatsuji, J. Chem. Theory Comput. 3, 605-618 (2007).
[3] On the color-tuning mechanism of Human-Blue visual pigment: SAC-CI and QM/MM study, K. Fujimoto, J. Hasegawa, S. Hayashi, and H. Nakatsuji, Chem. Phys. Lett. 432, 252-256 (2006)
[4]Origin of color tuning in human red, green, and blue cone visual pigments: SAC-CI and QM/MM study, K. Fujimoto, J. Hasegawa, and H. Nakatsuji, Chem. Phys. Lett. (2008) in press.