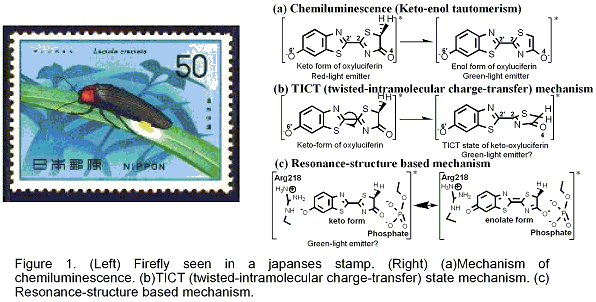
Firefly luminescence is intriguing and even mysterious photo-biological phenomenon. The firefly luciferase has also become important tool for bio-molecular imaging, because of the highly efficient conversion of chemical energy into light. In the case of North American Firefly (Photinus Pyralis), the luciferin is transformed into electronically-excited oxyluciferin (OxyLH2) inside the firefly luciferase enzyme (Luc), and exhibits the yellow-green emission. In chemiluminescence, the keto- and enol-isomers of OxyLH2 emit red (620 nm, 1.97 eV) and green (560 nm, 2.20 eV) lights, respectively (Figure 1 (a)). It has been established that this change is due to keto-enol tautomerism. Therefore, yellow-green light in the firefly luminescence had long been ascribed to the enol-form of OxyLH2. However, a recent research showed that 5,5-dimethylluciferin confined to the keto-form can exhibit the yellow-green emission. Alternative mechanisms were proposed: TICT (twisted-intramolecular charge-transfer) state mechanism (Figure 1 (b)) and resonance-structure based mechanism (Figure 1 (c).

In the present study, we determined the structure of the oxyluciferin-luciferase complex in the excited state by using the molecular-mechanical calculations and large-scale CI-Single calculations. SAC-CI calculations were performed to obtain precise excitation energy of the obtained structures. The present SAC-CI theoretical study consistently explains the color of the firefly fluorescence both in chemiluminescence and bioluminescence. The keto-form of oxyluciferin can emit both green and red lights in the firefly luciferase and in solution, respectively. Depending on the environment, the emission energy showed remarkable blue-shift. The keto-form of firefly luciferin shows the red light in the chemiluminescence observed in the solution (1.97 eV in experiment and 2.08 in SAC-CI theory). With the help of the protein environment and AMP, the emission energy remarkably shifts to higher-energy region and corresponds to the green light (2.23 eV in experiment and 2.13-2.33 eV in SAC-CI theory). The result of the analysis indicates that the origin of the blue-shift is in the electrostatic interaction between the oxyluciferin and the environment.
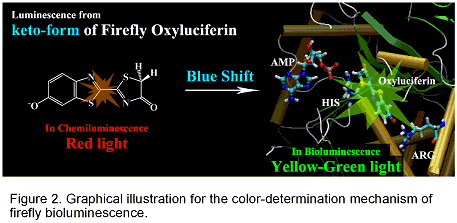
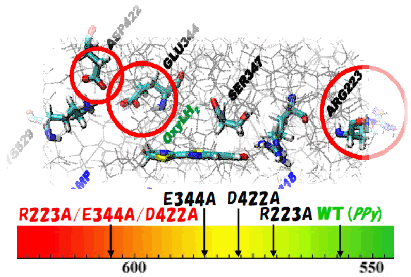
To identify amino acids which are important for controlling emission energy, we performed the symmetry-adapted cluster-configuration interaction (SAC-CI) calculations for the ground and excited states of firefly oxyluciferin-luciferase complex and analyzed the electrostatic interactions between oxyluciferin and the surrounding proteins. On the basis of the result of analysis, we propose mutations to artificially change the firefly luminescence color of Photinus pyralis. The present proposal is based on a modification of the electrostatic interactions by the mutation of the amino-acid residues of the luciferase proteins, Arg223Ala, Glu344Ala, and Asp422Ala mutations. Theoretical mutation simulations were performed to examine the proposals. SAC-CI method was used for calculating the ground and excited states of oxyluciferin. Compared with the wild type (2.23 eV, 557 nm, yellow-green), the combined triple mutant is expected to show reddish-orange luminescence (2.05 eV, 602 nm).
[1]Red Light in Chemiluminescence and Yellow-green Light in Bioluminescence: Color-tuning Mechanism of Firefly, Photinus pyralis, studied by the SAC-CI method, N. Nakatani, J. Hasegawa, and H. Nakatsuji, J. Am. Chem. Soc. 129, 8756-8765 (2007).
[2]Artificial Color Tuning of Firefly Luminescence: Theoretical Mutation by Tuning Electrostatic Interactions between Protein and Luciferin, N. Nakatani, J. Hasegawa, and H. Nakatsuji, submitted.